National Drugs Control System: guide for users
Updated 30 November 2023
Introduction
The National Drugs Control System (NDS) is an online facility designed to allow you to make applications for licences for the import and export of controlled drugs and precursor chemicals. It is also where licence holders can add preparations and overseas trading partners to their accounts. This guide will provide information on how to:
- register for an NDS account
- set up trading partners and preparations
- apply for import and export licences for controlled drugs and precursor chemicals
- endorse licences online
The Drugs and Firearms Licensing (DFLU) are available to provide assistance at any stage of the process, Monday to Friday, between 9:30am and 3:30pm. Our e-mail is dflu.ie@homeoffice.gov.uk and our telephone number is 0300 105 0248.
Please email us in the first instance or if you require any clarification regarding the user guide.
Email: dflu.ie@homeoffice.gov.uk
Telephone: 0300 105 0248
NDS registration
Domestic licence to trade
Before companies can register for an NDS account they must have a valid UK controlled drug or precursor chemicals domestic licence in place, covering the appropriate schedules for the substances they wish to import or export.
In most circumstances, a company which handles controlled substances under the Misuse of Drugs Act 1971 and Misuse of Drugs Regulations 2001 requires a controlled drugs domestic licence.
A company which handles precursor chemicals which are controlled under Regulation (EC) No. 273/2004 requires a precursor chemical domestic licence or registration, although this does depend on the chemical, activity and in some cases, quantities.
How to register for a controlled drug or precursor chemical licence
New and current licensees who wish to handle controlled substances should read our information on drugs licensing . This details all requirements, including the costs of registering and the costs of a licence.
The Drugs Domestic Licensing may be contacted on:
Email: dflu.dom@homeoffice.gov.uk
Telephone: 0300 105 0248
Applying for an NDS account
The NDS home page can be accessed via the .
New users should click the ‘Register Now’ button on the blue menu bar. This will take you to a user registration screen.
Complete all the relevant information, the email address that you register to your user account is where any licences will be sent to.
Username – this must be between 5-8 characters and not contain numbers or special symbols (@ etc).
Please check the ‘Allowed to Submit’ box if your company permits you to submit applications to the Home Office (if you do not select this box, you will only be able prepare draft for a colleague to then submit).
Once you have completed all the data, submit the request and email dflu.ie@homeoffice.gov.uk and let the team know you have submitted your request.
Please note
-
Do not apply for an NDS account unless you hold a domestic controlled drug or domestic drug precursor licence.
-
You will need an account for each site that controlled drugs or drug precursor will be shipped from or to.
-
The registered email address is where any issued import or export licences will be sent to (please ensure this is accessible to others in the event of your absence).
-
You cannot use the same email address for multiple accounts.
-
You must notify dflu.ie@homeoffice.gov.uk if you leave a company and/or no longer require an NDS account.
Logging on for the first time
Step 1
First time login only
Once your account has been approved you will receive an email within 24 hours providing you with your username. You may be prompted to reset your password. The requirements are:
- have at least one digit
- have at least one uppercase letter
- have at least one lowercase letter
- have at least one special character
- be from 10 to 32 characters in length
Step 2
If an account has a password expired then the system will prompt the user to reset the password.
Step 3
If the account is active and the password is valid then the system will log the user in.
Step 4
If the user has forgotten a password, then the user must recover it with the ‘forgot password’ option.
Process for requesting preparations or establishments
To apply for an import or export licence, you will need to have overseas trading establishments (these are referred to as ‘Foreign Trading Establishments on NDS) and if relevant, preparations (e.g., finished dose products) added to your account.
If you are shipping preparations, your licence application needs to match this. You must not select raw materials or pure substances in absence of a preparation in your account.
In the case of export applications, this will be apparent from the detail on the import licence e.g., an application to ship raw material against an import licence for finished dose medication will be rejected.
Step 1
Email your request to dflu.ie@homeoffice.gov.uk, with the subject “PREPARATION Request” or “FOREIGN TRADING STABLISHMENT”.
For preparations requests
Provide as much detail as possible, e.g., product name, dosage, base drug, quantity, format, pack size etc. See the examples below:
- Matrifen 50
- Fentanyl 5.5mg
- 5 patches / pack
If you know what brand you are shipping, it is important to apply for that brand. In some cases, products that have the same dosage or delivery can have different overall base drug quantities per unit, fentanyl patches are an example of this.
For foreign trading establishment requests
Provide as much detail as possible, e.g., company name and full address.
If your company has multiple sites, please indicate which site this should be added to. This should be indicated as the first line of the site address or town. Do not reference your user account.
Step 2
If we have it already set up on the wider NDS database, we will consider your request and advise you of the outcome. It will take up to 24 hours for any additions to show on your account.
Step 3
If it’s not set up, then we will let you know and ask you to register it on NDS (guidance below). You must reply by return with the request ID once you’ve created the request. Failure to do that will result in your request being cancelled. We will then consider the request and advise you of the outcome. It will take up to 24 hours for any additions to show on your account.
Creating favourites within Preparations
Within NDSWeb, you can create a list of Substance/Preparations ‘favourites’ to save scrolling through and selecting something from the exhaustive list.
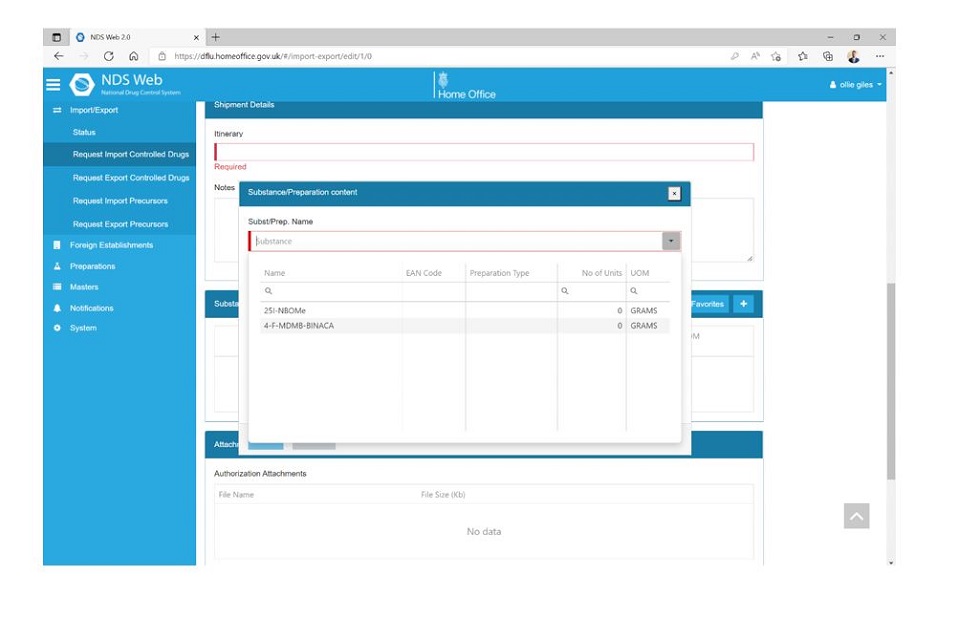
Step 1
Log into NDSWeb and select ‘Settings’ at the top right of the screen. If you navigate across to ‘User Settings’. You will see an option to enable Substance/Preparation Favourites. Select this and click ‘Save’.
Step 2
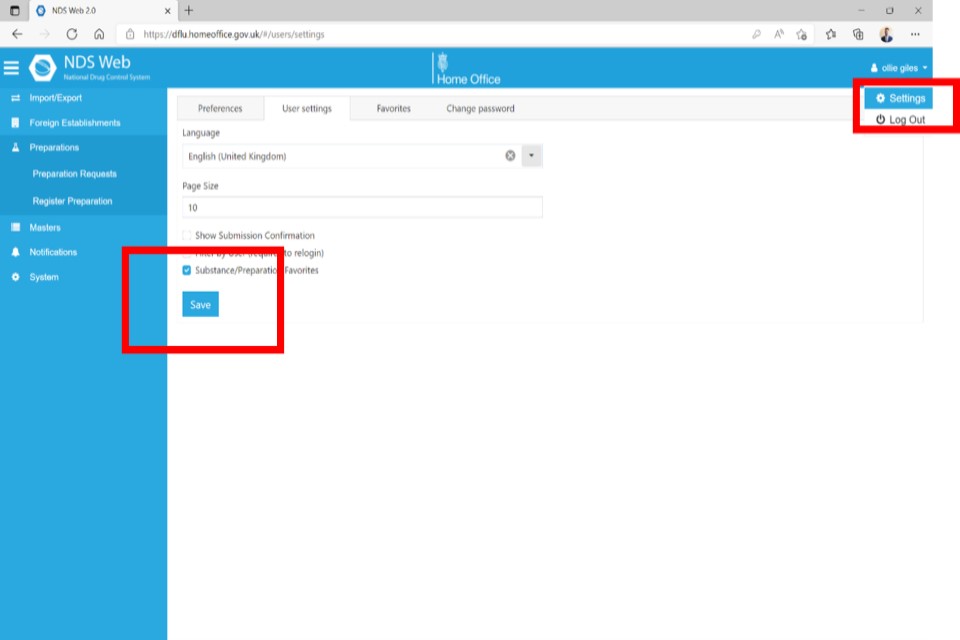
Use the blue arrows to add or remove Substances/Preparations to your Favourites list. When finished, click ‘Save’ at the bottom of the page.
Step 3
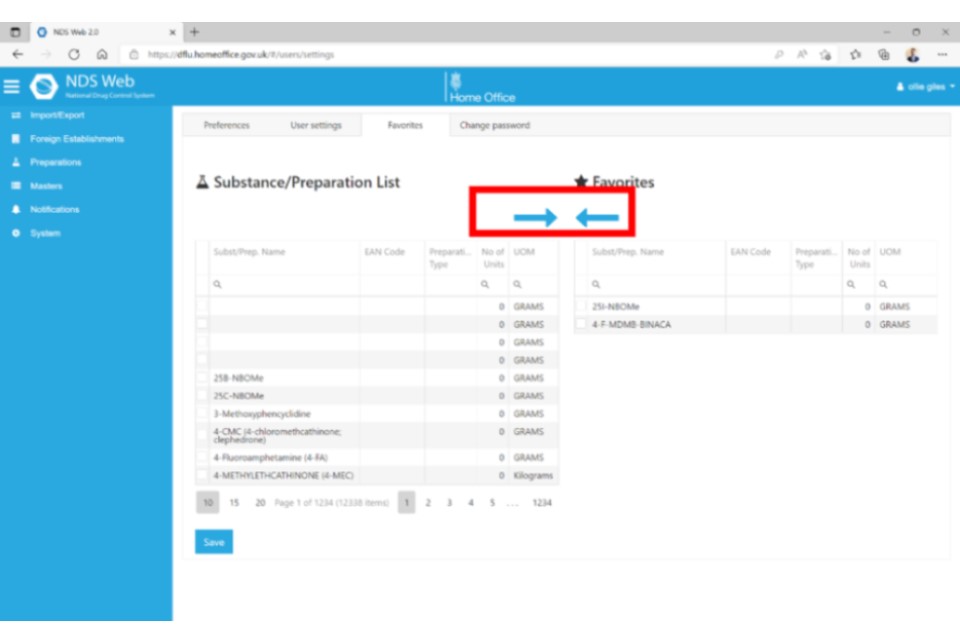
If you now begin a new ‘Request Import Controlled Drugs’ request, when you come to the Substance/Preparation Details, you will see a blue box that either says ‘Favourites’ or ‘All’. Make sure ‘Favourites’ is selected, then click the plus (+) icon to select from the dropdown list.
Step 4
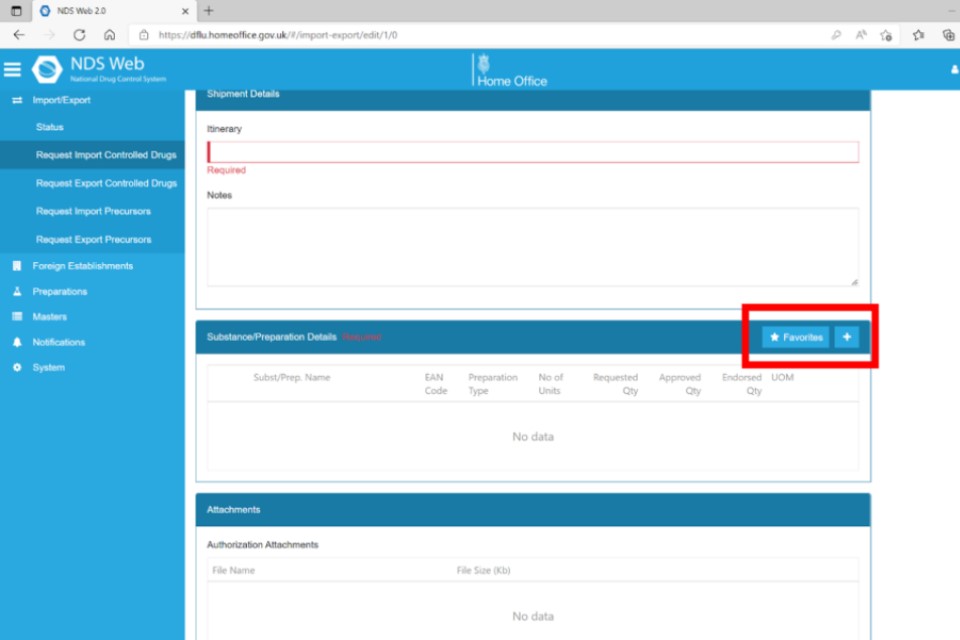
Your favourites will now appear in the dropdown menu for you to select.
Applying for trading partners
If advised that the trading partner you require does not exist on the system you must register them directly on NDS.
Click ‘Foreign Establishments’ on the blue menu bar.
Select ‘Register Foreign Establishment’.
Once you are in the ‘Foreign Establishment’ section:
-
select the country from the dropdown menu
-
fill out the name of the establishment
-
fill out the address details (for long addresses, you can add more information in the ‘street name line’ using a comma to separate each line)
-
ensure the postcode/zip code is entered into the ‘zip code’ section and not in any other sections
-
ensure the ‘city’ and ‘state’ sections are filled in
You can either save as a draft if you need to check something and come back to it later or submit the request.
Once you have submitted the request, reply on the email thread where the Import/Export team have authorised you to register the ‘Foreign Trading Establishment’ and advise the team of the request ID. We will consider your request and advise you of the outcome. It will take up to 24 hours for any additions to show on your account.
Applying for a Preparation
If advised that the preparations of drugs that you intend to trade in does not exist on the system, you will need to submit a request through NDS.
All substances are available already, but an application will not be granted if you do not have the correct domestic licence for the selected substance.
Select ‘“Preparations’ from the blue menu bar.
Click on ‘Register Preparation’.
Preparation
-
Preparation type: select the format of the product e.g., tablets, capsules, ampoules etc
-
Preparation name: enter the name of the product, this can be a brand name or generic (it must not reference the quantity/pack in the case of tablets, caps, injections, ampoules etc, however liquids in bottles should reference the total content in millilitres
-
Unit of measurement: how the preparation is packaged e.g., packs, bottles, barrels
Controlled substance
-
Substance name: enter the base drug
-
Quantity / volume: this should be in grams (NB this should be per dose, with the exception of any bottles which should represent the total amount in the bottle)
-
Click ‘Insert’ to add the record
Package size
-
Enter the number of doses per unit of measurement (UOM) (with the exception of liquids in bottles which should always be ‘1’)
-
It is not essential to add an EAN code
Submit the request (or save it as draft if necessary).
Once you have submitted the request, reply on the email thread where the Import / Export team have authorised you to register the ‘Preparation’ and advise the team of the request ID. We will consider your request and advise you of the outcome. It will take up to 24 hours for any additions to show on your account.
This information must be recorded accurately as this is the information that will appear on your licence.
If we have to cancel any applications, we will provide you with the reason.
Applying for an export licence for controlled drugs
Before starting your licence application, please ensure that you have already have the foreign trading establishment / preparation you require in your account.
Select ‘Import/Export’ from the blue menu bar.
Select ‘Import/Export’ requests from the dropdown menu which will take you into a new screen.
Select ‘Create Import/Export Request’ button.
Request type
From the dropdown, menu select ‘export controlled drugs’
General information
-
Type of permit: ‘single product’ or ‘multiple products’ (you can add up to 4 lines on each licence)
-
General notes: select whether the substances are for domestic use or for re-export in the country you are exporting to (you cannot submit a request for products that are for domestics use and for re-export on the same licence, they must be split across 2 licences). It is important to record this accurately as substances that are for re-export do not come off a country’s estimates
Exporter details
This will be pre-populated with your licensed site details.
Importer details
-
Country: select the country you are exporting to
-
CA Department: select the CA from the drop down or leave this blank
-
Establishment: select the establishment you wish to ship to from the dropdown (if this is not visible this will need to be added before you can submit the request)
Substance/Preparation details
-
Substance/Preparation name: click ‘add new record’ and then select the raw material/API substance or finish dose preparation you are shipping from the dropdown menu (please note that only the preparations you have in your account will be visible). All users have access to all the raw materials, but you must not select raw material when shipping finished dose medications. Any such applications will be rejected
-
Quantity: please select the quantity you require NB this is will in grams for raw material or a defined unit of measurement e.g., if you have Pregabalin 50mg in packs of 56 set up, you request your quantity in packs. Click ‘Insert’ and then you can repeat the process to add further lines
-
You can go back in and edit any of these details whilst the licence application is in draft mode. You will not be able to change any details once the licence has been submitted
Attachments
You must attach an import permit or letter of no objection (LONO) from the relevant Competent National Authority to your export application.
Comments
You can add supporting comments or statements here and these will appear on the licence. These need to be agreed by DFLU.
Final steps
You can save your licence application by clicking the ‘save as draft’ button and access it later – this is useful if you need to check any information before submitting the licence.
Once you are satisfied that your application is complete you can click the ‘Submit Request’ button.
You can check the status of your request by clicking ‘Import/Export’ from the blue tool bar at the top of the main NDS screen and then selecting ‘Import/Export Requests’ from the dropdown menu. This will show a list of your requests and its status. For more information, you can click on ‘more …’ at the end of each request.
Once a licence has been submitted it will show as ‘Requested’ in the request status screen. If you then need to cancel a licence, please email dflu.ie@homeoffice.gov.uk with the web ID and a member of the team can cancel it for you.
Once a licence is ‘In Review’ or ‘Approved’, it cannot be cancelled and the licence fee will be charged.
Applying for an import licence for controlled drugs
This process for applying for an import licence is very similar to applying for an export licence.
Before starting your licence application, please ensure that you have already have the foreign trading establishment or preparation you require in your account.
Select Import/Export’ from the blue menu bar.
Select ‘Import/Export’ requests from the dropdown menu which will take you into a new screen.
Select ‘Create Import/Export Request’ button.
Request type
From the dropdown menu, select ‘import controlled drugs’.
General information
-
Type of permit: select either ‘single product’ or ‘multiple products’ (you can add up to 4 lines on each licence
-
General notes: select whether the substances are for domestic use or for re-export from the UK (you cannot submit a request for products that are for domestics use and for re-export on the same licence, they must be split across 2 licences). It is important to record this accurately as substances that are for re-export do not come off the UK estimates
Importer details
This will be pre-populated with your licensed site details.
Exporter details
-
Country: select the country you are importing from
-
CA department: select the CA from the drop down or leave this blank
-
Establishment: select the establishment you wish to ship from in the dropdown (if this is not visible this will need to be added before you can submit the request)
Substance/Preparation details
-
Substance/Preparation Name: click ‘add new record’ and then select the raw material/API substance or finish dose preparation you are shipping from the dropdown menu (please note that only the preparations you have in your account will be visible). All users have access to all the raw materials, but you must not select raw material when shipping finished dose medications, any such applications will be rejected. It is advisable to confirm an import licence request is for raw material in the user comments section to avoid delay cause by queries from the Import/Export team
- Quantity: please select the quantity you require NB this is will in grams for raw material or a defined unit of measurement e.g., if you have Pregabalin 50mg in packs of 56 set up, you request your quantity in packs. Click ‘Insert’ and then you can repeat the process to add further lines
- You can go back in and edit any of these details whilst the licence application is in draft mode. You will not be able to change any details once the licence has been submitted
Attachments
You will generally not need to attach any documentation to an import licence application, exceptions to these are MHRA notifications for CPBM imports.
Comments
You can add supporting comments or statements here and these will appear on the licence. These need to be agreed by DFLU.
Final steps
You can save your licence application by clicking the ‘save as draft’ button and access it later – this is useful if you need to check any information before submitting the licence.
Once you are satisfied that your application is complete you can click the ‘Submit Request’ button. You can check the status of your request by clicking ‘Import/Export’ from the blue tool bar at the top of the main NDS screen and then selecting ‘Import/Export Requests’ from the dropdown menu. This will show a list of your requests and its status. For more information, you can click on ‘more …’ at the end of each request.
Once a licence has been submitted it will show as ‘requested’ in the request status screen. If you then need to cancel a licence, please email dflu.ie@homeoffice.gov.uk with the web ID and a member of the team can cancel it for you. Please note that once a licence is ‘In Review’ or ‘Approved’, it cannot be cancelled, and the licence fee will be charged.
Applying for an import permit for precursor chemicals
Import licenses are only required for Category 1 precursor chemicals.
The process for applying for an import licence for precursor chemicals is again largely similar to applying for a controlled drugs import licence. However, for precursor chemicals the maximum number of substances that can be added to an application is 2.
Applications must be made either by the named responsible officer or guarantor, both of whom should have NDS accounts. Where any licence is requested on their behalf, notes must be added in the ‘user comments’ section to confirm they are aware of application.
You must attach a copy of your Category 1 precursor chemical domestic licence to the application as well as a completed ‘Import PC Questionnaire & Checklist’ document (you can obtain a copy of this from dflu.ie@homeoffice.gov.uk).
Please include information on the ultimate consignee, broker, carrier and manufacturer, if known, as it will aid our processing of the application.
Applying for an export licence for precursor chemicals
The process for applying for an export licence for precursor chemicals is again largely similar to applying for an export licence for controlled drugs. However, the process will take longer as DFLU need to obtain consent from the competent authority of the importing country.
This is known as “pre-export notification” (PEN) and takes 17 working days. Once this time has passed and provided there are no objections from the importing country, the licence will be granted. We undertake all PEN actions for you, you do not need to do anything additional other than apply for a licence on NDS and factor in timings in your export planning.
Overall you should allow 20 working days for an export precursor chemicals licence application to be considered (this allows for time to review the application, input the proposed export onto PEN, the clearance time, and if approved by the overseas competent national authority, the licence issue). Any queries can significantly add to this time so please provide all the required information.
Please note the following:
You must attach the corresponding import licence and/or make reference to it in the ‘Import Authorisation Document’ section.
Some countries do not issue individual import licences for each shipment of precursor chemicals and instead issue an annual ‘blanket’ licence.
Ultimately, it is your responsibility to obtain as much information as you can from the importer as queries from overseas Competent National Authorities (CNA) through the PEN system can significantly add to the lead-time for export licences.
In some cases where not enough information is provided, overseas CNA’s will object to the shipment on PEN and the process will need to be restarted with a fresh application on NDS.
You must attach a copy of your domestic precursor chemical licence to the application.
Receiving import/export licences
When a licence application has been approved or returned to the user for correction or cancelled, a notification e-mail will be sent to the e-mail address registered on the user account.¬Ý For applications cancelled or returned to user for correction, explanation can be found within the comments section in the licence application.¬Ý This can be found in the Import / Export ‚Äì Status menu bar.
For all approved licences any user assigned to the company account will be able to log into NDSweb, view all requests and then download each individual licence.
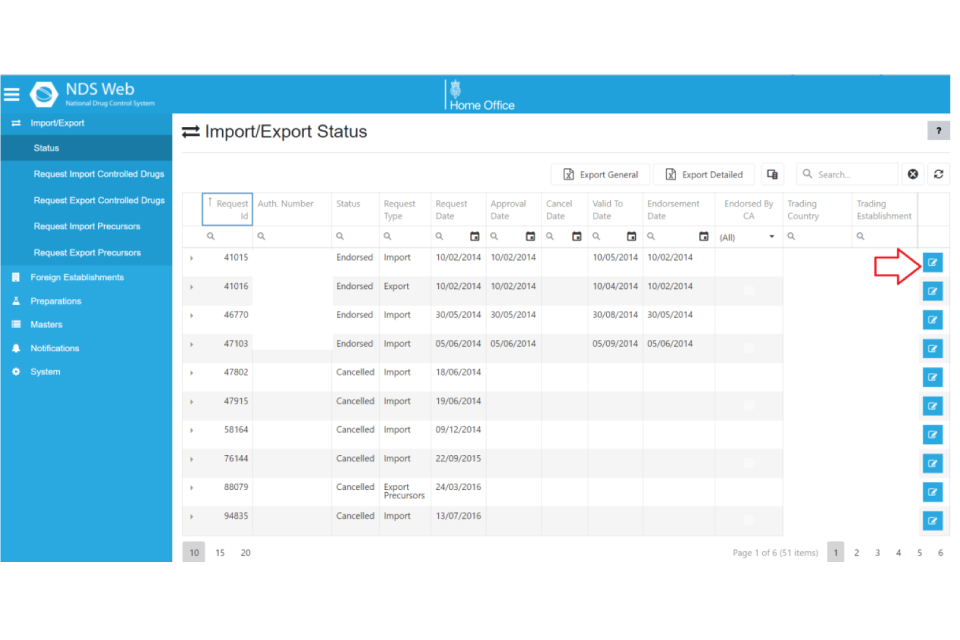
The process to download a licence;
- log into your NDS account
- select “import / export” from the blue menu bar
- select “status”
- click “more ….” at the end of the licence you want to view.
- scroll to ‘Attachments’ where the PDF licence will be available to download
Endorsing licences
Part of your obligations as a holder of a domestic licence for controlled drugs or precursor chemicals is endorsing the quantity of goods shipped on each licence.
The Home Office, as the United Kingdom Competent Authority, is a signatory to the UN Single Convention on Narcotic Drugs 1961, the Convention on Psychotropic Substances 1971 and the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances, 1988.
Through these, the UK is allocated an agreed yearly limit (the “estimate[1]”) for controlled drugs and precursor chemicals that can be imported. The same process is applied globally, so when exporting we have regard to the ‘estimates’ for the receiving country in our considerations.
As such, you are required to upload the endorsement immediately after the shipment has been sent or received or on the expiry date of the licence, if it is not used. This is a condition of all licenses issued.
Timely endorsement of licences maintains the integrity of the estimate system for all licensees, allows DFLU to process licence applications as quickly as possible, and enables the UK to meet its international obligation under UN conventions. Any delays with endorsements will result in a suspension of your account until they have been cleared.
How to endorse a licence
After shipment, the bottom section of Copy 1 of your licence must be completed. The bottom section of the Copy 1 licence is shown below:
Following shipment, you must detail below the actual amount of each substance/preparation shipped and endorsed online:
1.
2.
3.
4.
Date of shipment:
Completed by (name):
Contact telephone number:
You can complete the shipment section ready for endorsement on Copy 1 of your licence. You can annotate the PDF electronically.
You must endorse the licence in the unit of measurement it was issued in. For example, if the licence was issued for packs of a finished dose preparation, you must endorse it in packs, rather than the base drug quantity.
-
Log into your NDS account
-
Select “Import/Export” from the blue menu bar
-
Select ‘Import/Export Endorsements’
-
Click ‘more ….’ at the end of the licence you want to endorse
-
Enter the endorsement date which is¬Ýthe date the shipment occurred not the date when you are submitting the figure (this must be within the validity of the licence).¬Ý If you did not use the licence, select the last day of the validity period.
-
Attach the endorsed licence (in most cases, if a licence is not attached, the endorsement will be rejected)
-
The Importer/Export details will be pre-populated
-
In the ‘Substance /Preparation’ section you will see the details of the approved licence. Click on ‘Edit’
-
Add in the quantity you either shipped or received. This should be in the unit of measurement that the licence was issued in. If you did not use the licence, please record the quantity as 0
-
Click ‘update’ and repeat the process for any additional lines you need to endorse
-
In the ‘user comments’ section, please add any narrative that would support your endorsement
-
You can either ‘save as draft’ or ‘submit’. Once submitted you cannot alter any detail on the endorsement
Please note
You can check the status of your endorsement in the ‘Import/Export Endorsements Status’. This will be either:
- ‘Approved’ (endorsement still to be submitted)
- ‘Endorsement Submitted’ (waiting to be reviewed by DFLU)
- ‘Endorsed’ (endorsement approved by DFLU)
- ‘Cancelled’ (endorsement has been cancelled by DFLU)
If we need to cancel the endorsement, we will provide some detail in the ‘CA Comments section’. This will then need to be endorsement manually, which is time consuming for all parties to please make every effort to submit a full, accurate and timely endorsements.
Manual endorsements
This needs to be completed by a member of the Import/Export team.
Please email dflu.ie@homeoffice.gov.uk with a copy of your endorsed licence, stating the date of endorsement and the quantity. We will confirm when this has been completed.
Please note that the status of the endorsement will always remain as ‘Cancelled’ even after it has been manually endorsed. It is therefore recommended that you retain the email that confirms the endorsement has been completed manually.
